CyaninPlus is backed by solid scientific research including:
- Antioxidant assays demonstrating its ability to neutralize free radicals and inhibit reactive oxygen species (ROS) formation*
- Research on cellular processes relating to healthy immune function and the support of a healthy inflammatory response*
- Human clinical trials indicating CyaninPlus' effectiveness in helping to maintain a healthy inflammatory response and in reducing discomfort associated with routine physical activity*
Antioxidant assays
CyaninPlus was tested in a panel of chemical and biological assays to examine its complex role in helping to reduce the level of free radicals.* The tests showed that CyaninPlus:
- Has antioxidant capacity*
- Provides antioxidant protection of live cells by neutralizing existing free radicals*
- Reduces the production of new free radicals by inflammatory cells under oxidative stress*
Importantly, cellular effects were seen at very low doses of CyaninPlus, which indicates that similar effects could be achieved through reasonable human dosages.
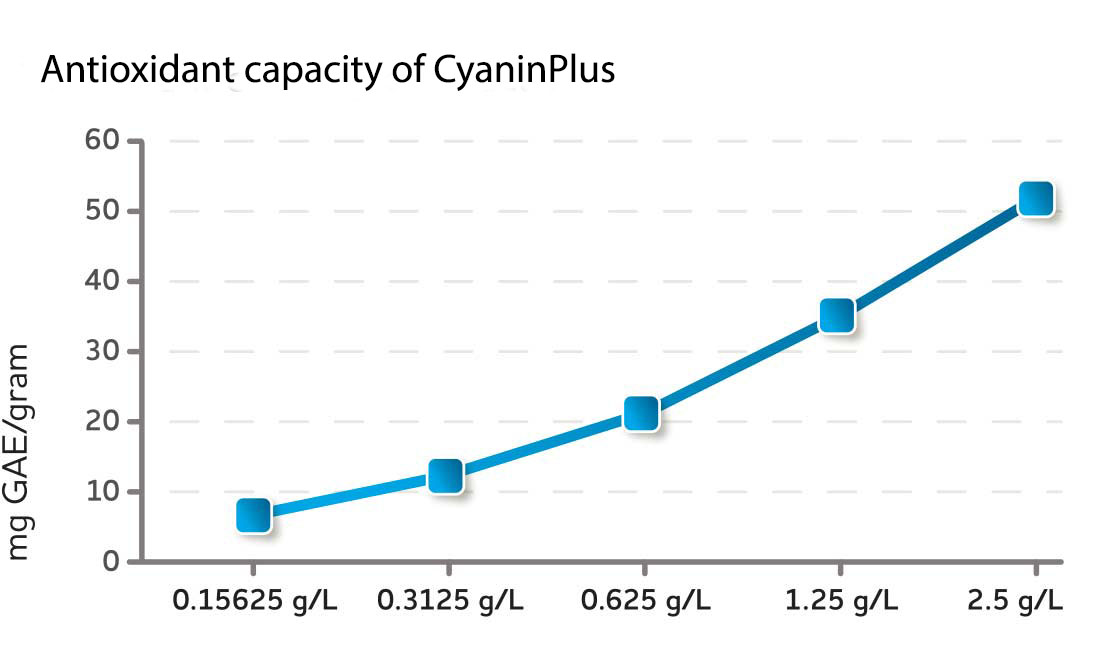
The antioxidant capacity of CyaninPlus was tested in the Folin-Ciocalteu assay, which is widely used to test and compare antioxidants in fruit, berries, wine, and many nutritional products. The antioxidant capacity of CyaninPlus exceeded that of an 80% pure phycocyanin-rich extract, suggesting that antioxidant compounds other than PC contribute to the total antioxidant capacity of CyaninPlus.* When the non-phycocyanin fraction was tested, it had an even higher antioxidant capacity per mg GAE/gram of dried material than PC alone.*
Fig. 1 CyaninPlus' total antioxidant capacity was measured using the Folin-Ciocalteu assay
The cellular antioxidant protection of CyaninPlus was evaluated using the CAP-e (cell-based antioxidant protection in erythrocytes) bioassay, which is a method for testing the bioavailability of antioxidants at the cellular level. Recognizing that many natural substances show strong antioxidant activity in vitro, but may not be bioavailable, CAP-e assesses the antioxidant potential of a substance to protect live cells from oxidative damage. CyaninPlus was shown to provide strong antioxidant protection to erythrocytes (red blood cells) in this model.* Both phycocyanin and the non-phycocyanin fraction of CyaninPlus actively provided cellular protection.*
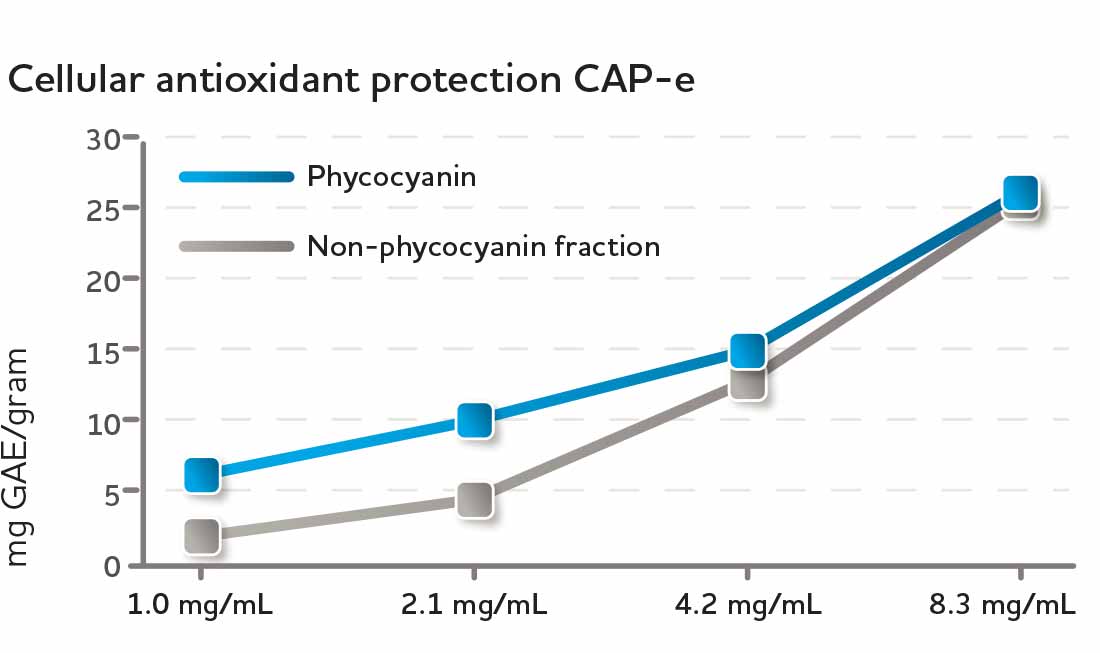
Fig. 2 The cellular antioxidant protection of CyaninPlus was evaluated using the CAP-e bioassay. Both phycocyanin (dark blue) and the non-phycocyanin fraction (gray) contributed robustly to the cellular protection from free radical damage.*
Peripheral blood polymorphonuclear (PMN) cells from healthy donors were used to test CyaninPlus' free radical inhibitory effect in a cellular model. The cells were exposed to an inflammatory insult, which would normally cause the formation of free radicals. However, inflammatory cells pre-treated with CyaninPlus reduced their formation of free radicals compared to control cells. Pre-treating cells with CyaninPlus caused a clear dose-dependent inhibition of the formation of Reactive Oxygen Species (ROS)—the higher the dosage, the greater the protection.* Importantly, treatment with CyaninPlus did not completely shut down free radical production. In addition, the effect was measurable at a dose much lower than that needed to cause an antioxidant effect suggesting that CyaninPlus has additional inflammation modulating roles beyond a direct antioxidant effect.*
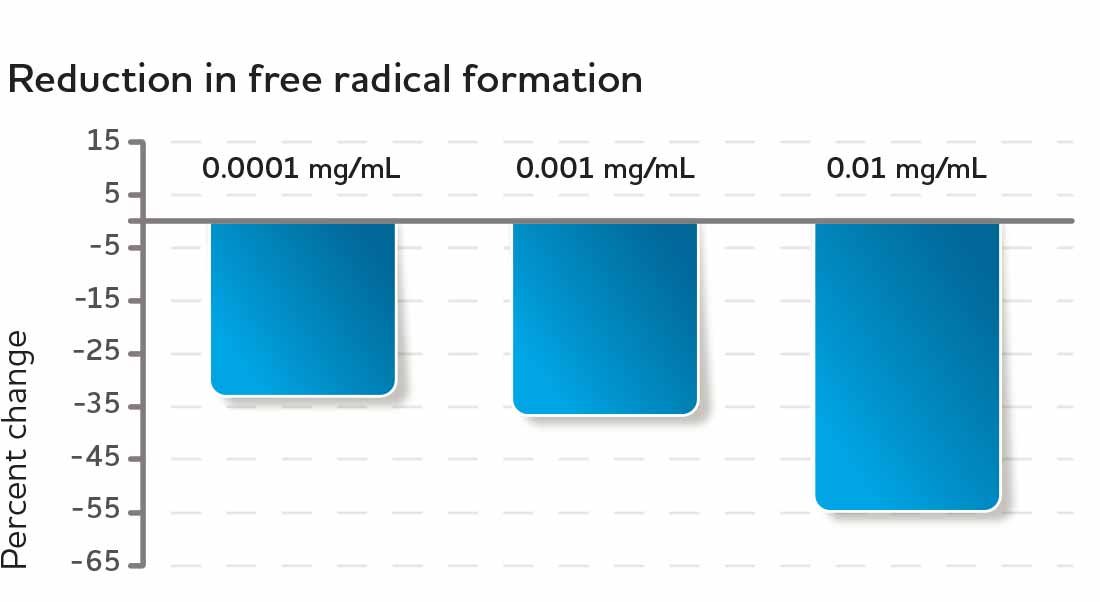
Fig. 3 Cells exposed to CyaninPlus produced fewer free radicals than cells not exposed, which suggests CyaninPlus has a cell regulatory function in addition to its antioxidant function
Cellular processes supported by CyaninPlus
COX-2 inhibitors have therapeutic applications because they help modulate inflammation. However, the cellular effects of some COX-2 inhibitors also disturb natural processes involved in mucosal tissue integrity and regeneration, as well as immune surveillance. That means they can do more harm than good.
CyaninPlus does effectively inhibit the activity of COX-2 enzymes.* At the same time, though, data has shown it also enhances specific tissue regenerative factors that are suppressed by some COX-2 inhibitors.* A panel of assays was therefore applied to examine the effects of cell exposure to CyaninPlus. The panel demonstrated that CyaninPlus has the following effects on human cells from healthy blood donors:
- Supports the production of granulocyte-macrophage colony stimulating factor (GM-CSF), a growth factor involved in intestinal immune and inflammatory responses.*
- Has a selective effect on cellular migration, in contrast to some nondiscriminatory COX-2 inhibitors.*
CyaninPlus increases production of GM-CSF
GM-CSF is a growth factor that promotes the integrity and repair of the epithelial barrier, the layer of cells lining the intestines. Studies indicate that GM-CSF plays an important role in the regulation of intestinal immune and inflammatory responses. When healthy lymphocyte cultures were exposed to CyaninPlus in laboratory cultures, cellular activation resulted in induction of GM-CSF production.*
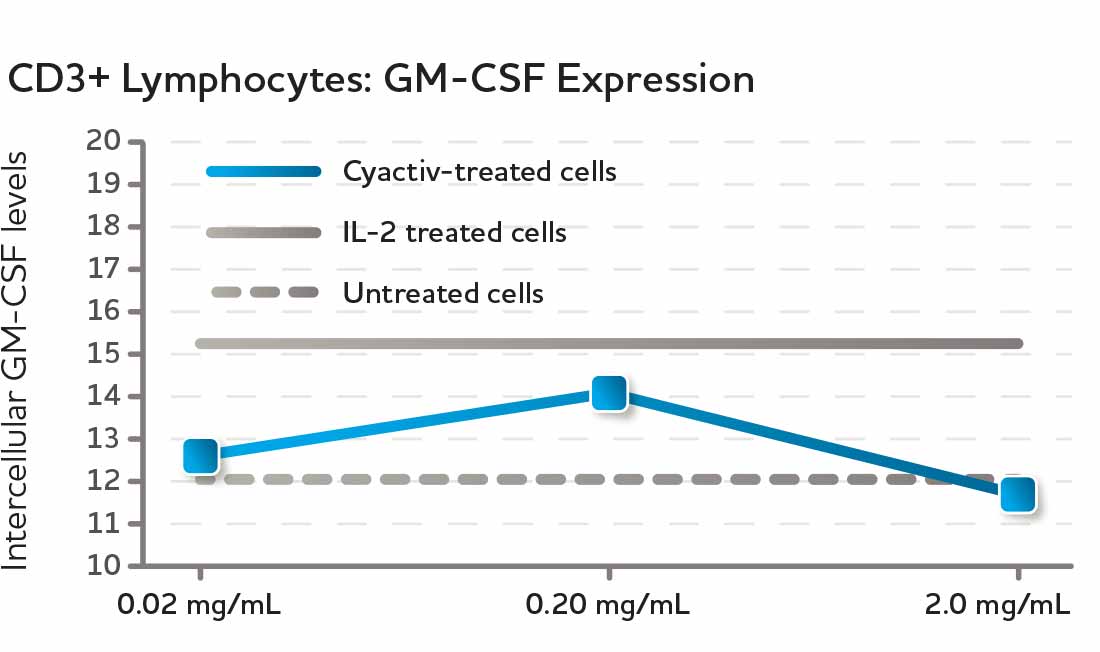
Fig. 4 Treatment of healthy human lymphocyte cultures with CyaninPlus results in increased production of GM-CSF. GM-CSF is a growth factor involved in maintaining healthy gastric mucosa and epithelial barrier integrity.
CyaninPlus affects migration of immune cells
Immune cell migration is a critical part of our immune surveillance program, because it ensures that immune cells are in the right place at the right time to protect us from harm. However, when immune cells migrate to the site of injury, they contribute to an inflammatory process that can cause more harm than good.
Polymorphonuclear (PMN) cells play a role in immune surveillance as well as infiltration into sites of inflammation. In circulating blood, PMN cells account for 70% of white blood cells and are the first line of defense against harm.
Some COX-2 inhibiting compounds are non-selective, meaning that in addition to inhibiting the harmful migration of immune cells (such as PMN to the site of an inflammatory insult), they also inhibit the helpful migration of immune cells as part of healthy immune surveillance.
CyaninPlus is different. Laboratory research reveals that CyaninPlus supports healthy cellular migration as part of immune surveillance, and selectively and partially inhibits migratory behavior in response to an inflammatory insult.* In a normal culture environment without inflammatory stimuli, CyaninPlus treatment resulted in increased PMN-related immune surveillance activity in immune cells harvested from healthy blood donors. In contrast, in the presence of an inflammatory chemoattractant, CyaninPlus reprogrammed the same cell type to a reduced migratory behavior.*
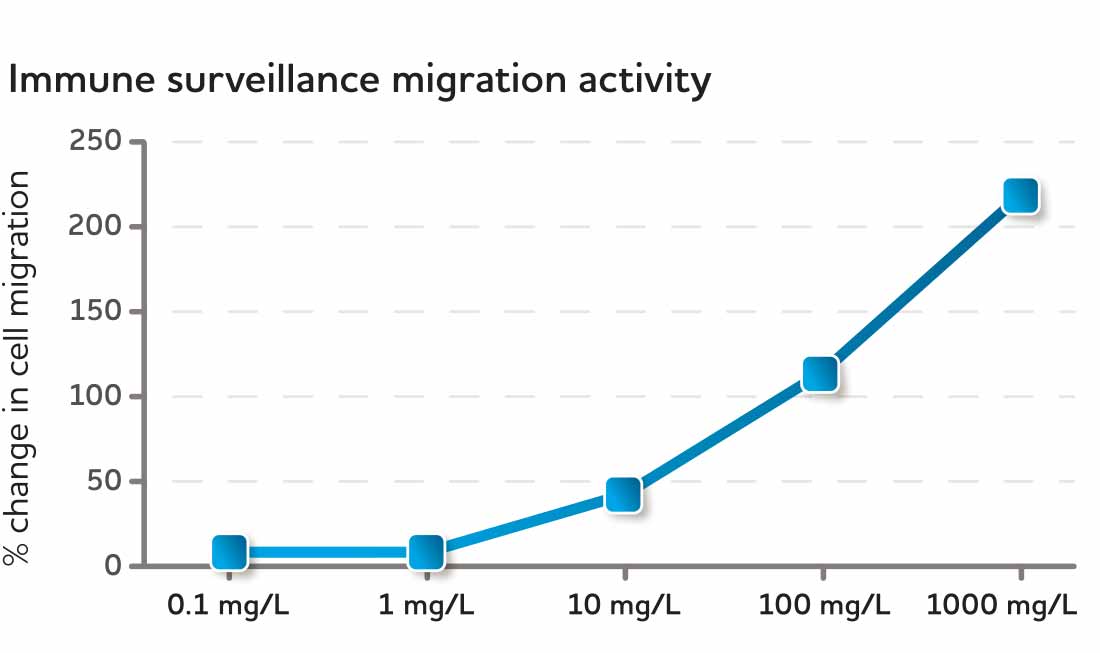
Fig. 5 Uninhibited migration of PMN immune cells following CyaninPlus administration
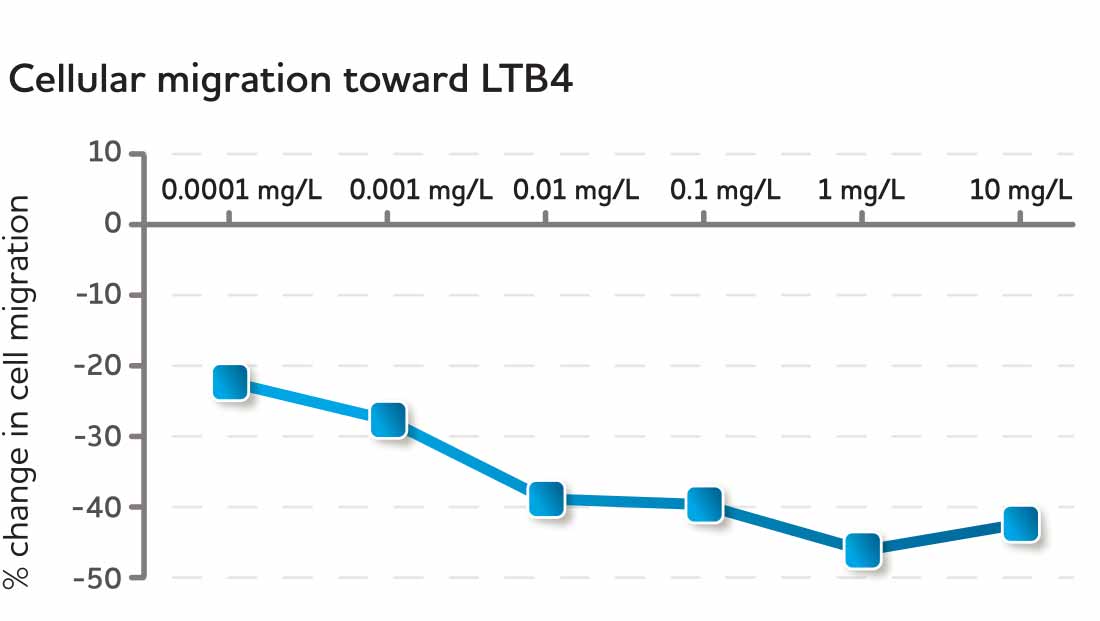
Fig. 6 Selective inhibition of cells in response to inflammatory mediators following CyaninPlus administration
Human clinical trials
Physical discomfort can inhibit general activity and lead to a more sedentary existence, which has health drawbacks. Three clinical studies have assessed the effect of CyaninPlus on physical discomfort:
- Study I: Open-label pilot study (1 gram daily dose)
- Study II: Placebo-controlled dose study, using a cross-over design (500mg, 250mg, placebo)
- Study III: Placebo-controlled, parallel-arm study on safety and comfort during physical activity (1 gram daily dose).
Participants, from healthy populations, in all three studies had mild-to-moderate discomfort in specific areas of the joints and muscles during physical activity. Researchers assessed scores for each person's areas of primary and secondary discomfort during physical activity. Significant improvement in comfort during physical activity was seen in all three studies. The data showed that CyaninPlus:
- Increased comfort during physical activity*
- Increased ability to perform various activities for daily living
- Improved quality of sleep*
- Increased physical energy levels*
As part of Desert Lake Technologies's commitment to ongoing research on CyaninPlus, pilot work is currently underway to evaluate its acute effects. Current pilot data suggests that CyaninPlus has acute effects on inflammatory markers and regenerative cytokines.*
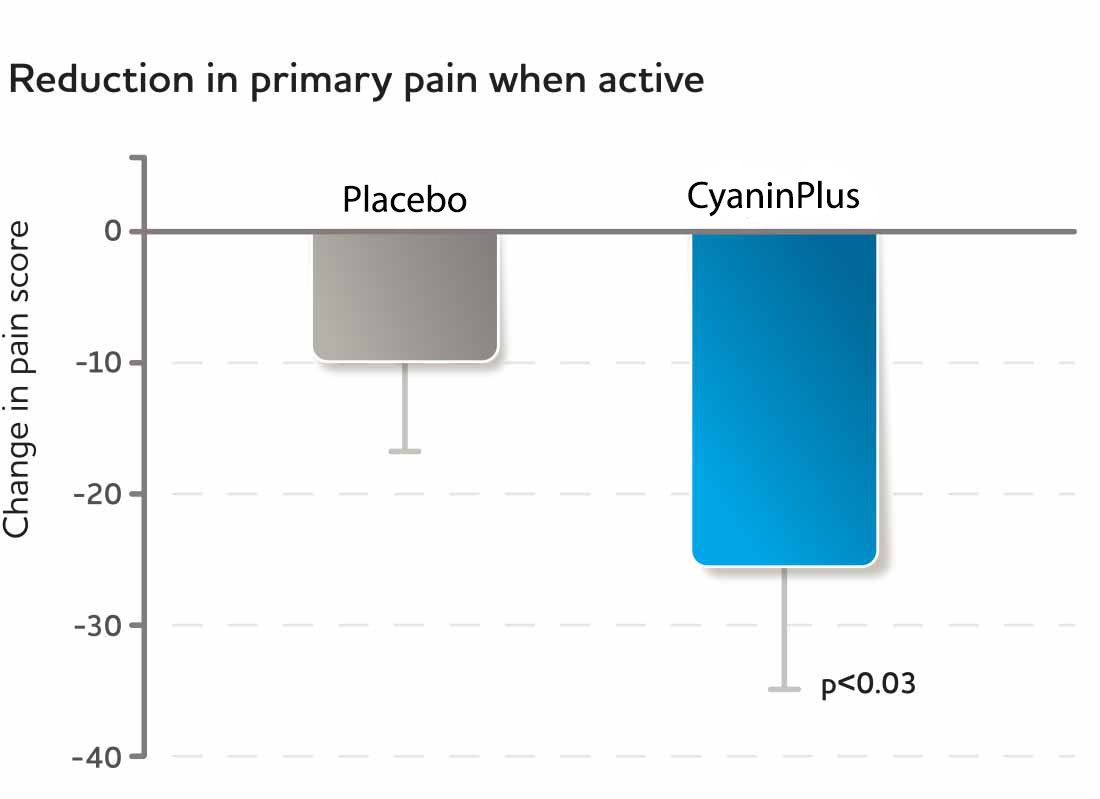
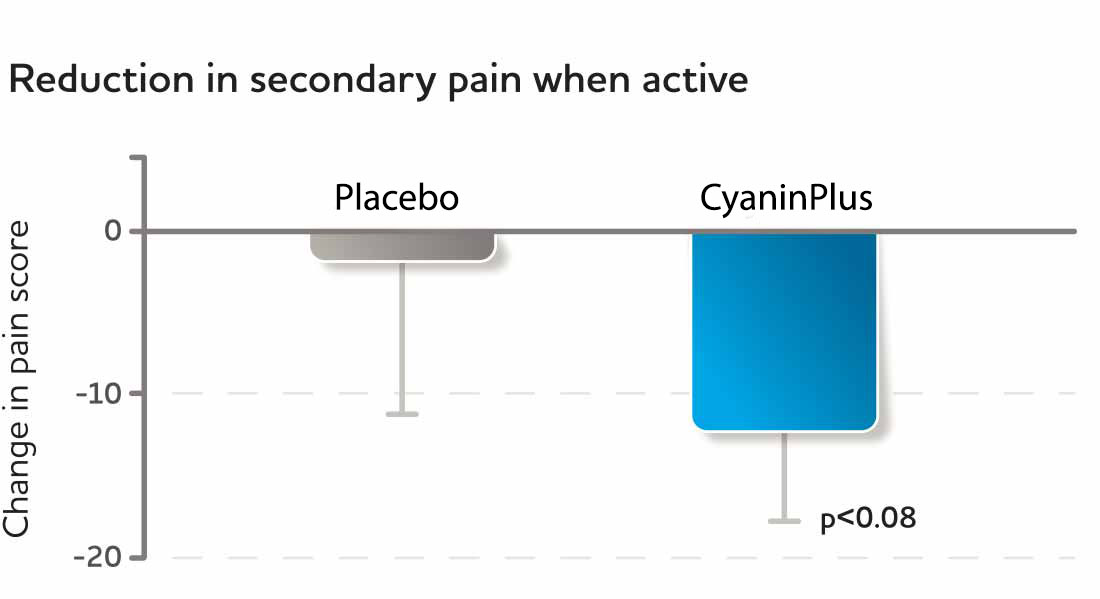
Fig. 7, 8 The two graphs above show the reduction in discomfort scores during physical activity after 2 weeks of consuming either placebo or CyaninPlus (1 gram/day).
In a randomized, double-blind, placebo-controlled parallel-arm study (Study III), participants showed highly significant reduction of physical discomfort as early as two weeks.*
Each study participant's anatomical area of primary and secondary discomfort was identified at the study's start and tracked at later visits. Discomfort was scored using visual analogue scales (VAS), where “0” indicated no discomfort and “100” indicated a great deal of discomfort. Subjects' increased feelings of comfort during physical activity was statistically significant for the primary complaints (p<0.03) and reached a statistical trend for secondary complaints (p<0.08).*